Natureza Develops Antimicrobials Against Candida Auris & Pseudomonas.
We will be taking this exciting new data to the 2023 Johnson & Johnson and BARDA BLUE KNIGHT ™ Symposium in Boston presenting data on two of our novel anti-infectives.
June 4, 2023 in Boston, Massachusetts
Natureza Products, Inc. is at the ESCMID/ASM Conference on Drug Development in Dublin presenting data on two of our novel anti-infectives.
The 2022 ASM/ESCMID Joint Conference on Drug Development to Meet the Challenge of Antimicrobial Resistance
October 4-7, 2022 in Dublin, Ireland
Natureza have tested their antimicrobials against two of the biggest emerging threats to medicine, Candida auris and Pseudomonas, and shown that their products kill both of these organisms with no resistance seen by either. Natureza have developed new formulations so that the antimicrobials canm be inhaled into the lungs, used on the skin or encapsulated for the intestine meaning that we can treat a wider range of infections than ever before. This year’s Symposium will be hosted in association with the 2023 BIO International Convention. Location: Omni Boston Hotel at the Seaport, 450 Summer St., Boston, MA. | Conference Website
Preclinical evaluation of a novel membrane-intercalating agent shows antibacterial activity with no generation of bacterial resistance.
Preclinical evaluation of a novel membrane-intercalating agent shows antibacterial activity with no generation of bacterial resistance
Membrane fluidity is key to the function of prokaryotic and eukaryotic cells. We have developed chitosan-coated esters of dodecanoic acid as membrane intercalating agents with anti-inflammatory and antibacterial properties. These can be targeted to Gram-positive or negative bacteria by alteration of charge and decoration on the chitosan coat, and bacteria show no innate or evolved resistance to these agents on exposure.
Background
We have observed previously that modification of bacterial membranes by the insertion of lipids with cyclic or branched moieties causes an increase in membrane stability and in bacterial heat tolerance. Where bacteria are unable to alter these lipids their ability to adapt becomes compromised as an increase in stability causes decreased fluidity and ability of the membrane to function.
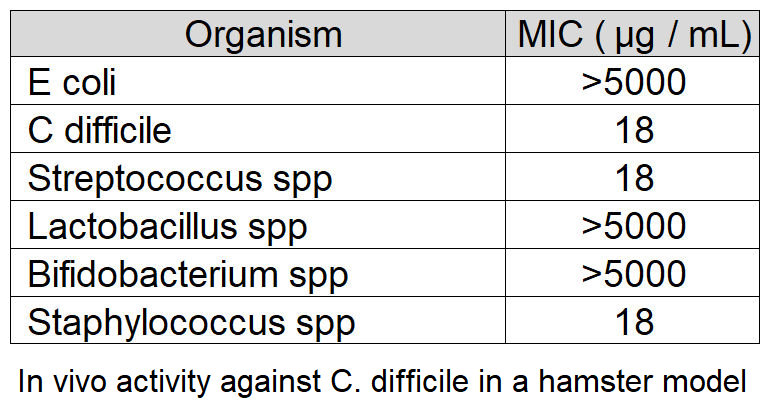
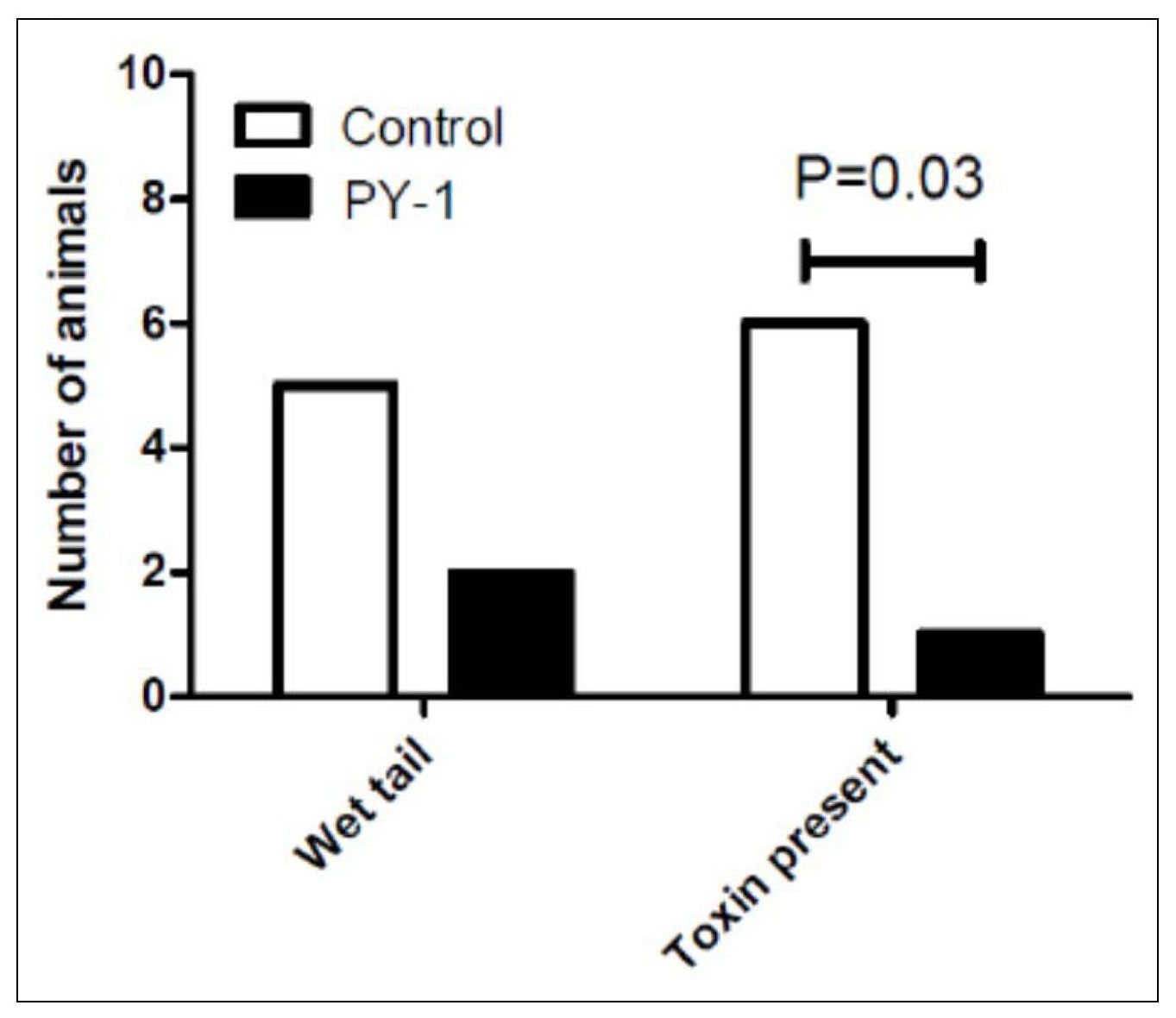
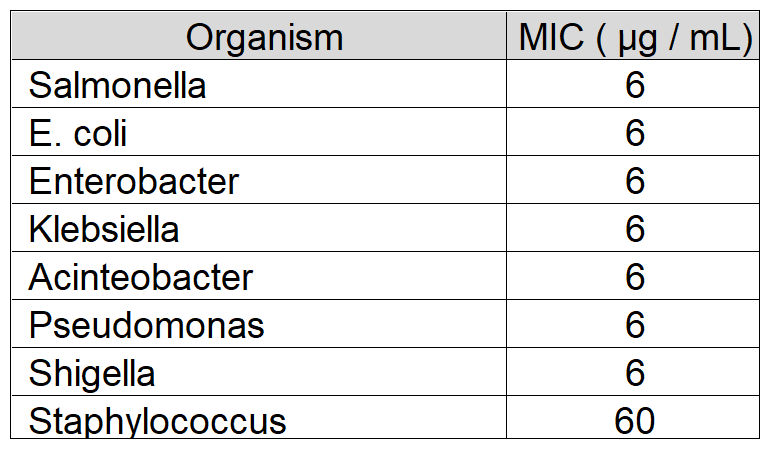
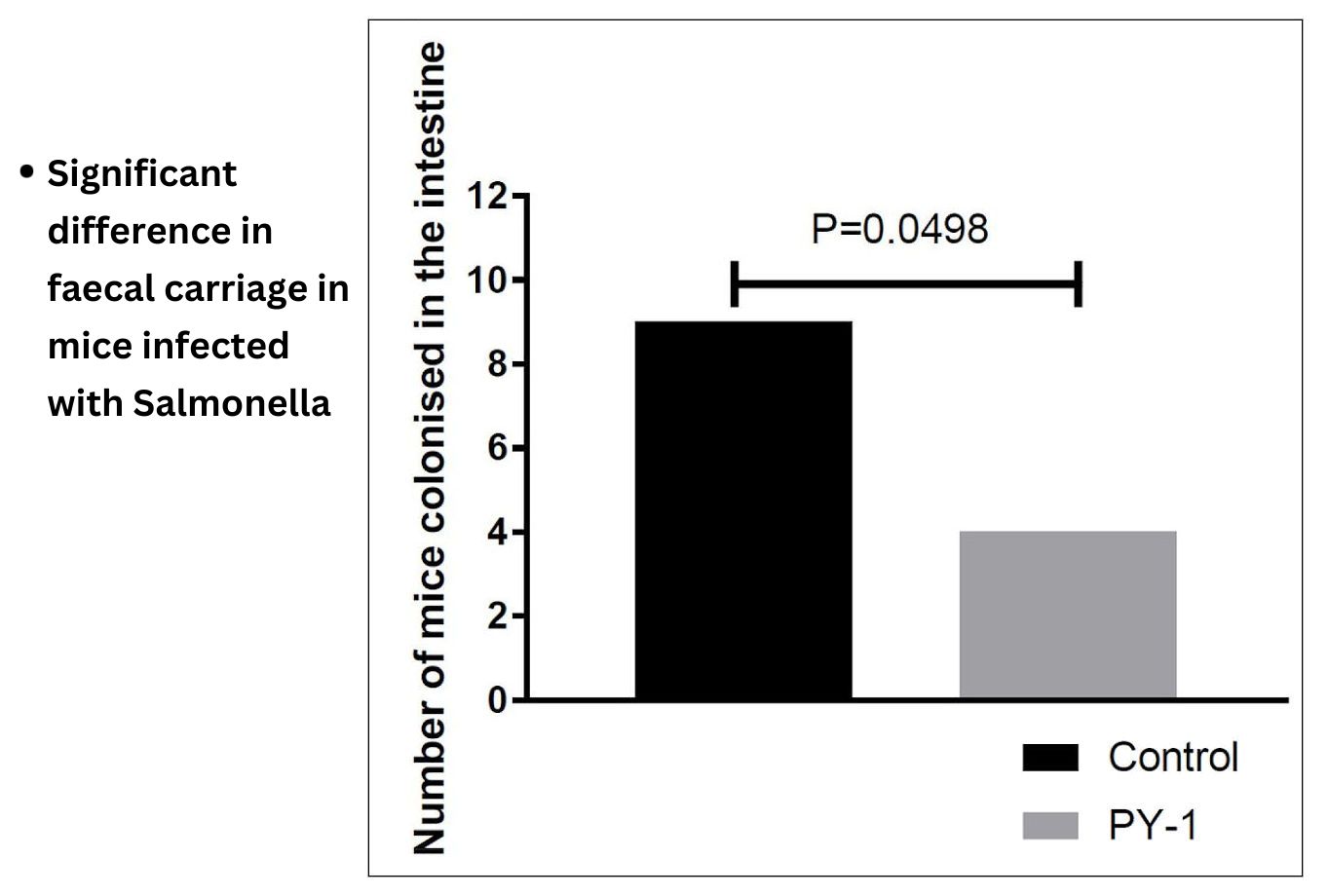
Natureza Products, Inc. has developed these lipids into orally dosable chitosan-coated particles (PY-1 for Gram positives and PY-3 for Gram negatives) that show activity against many of the WHO priority pathogens and have also shown that these are active in in vivo models.
PY-1